CASE STUDY – ADA ASSAYS
Bispecifics (Bs) are biopharmaceutical products that bind to two different targets (epitopes). These are mainly two types: IgG-like (antibodies Abs) and non-IgG-like which include Bites (bispecific T-cell engager) and DARTs (Dual-affinity Re-targeting Antibody).
There are currently over 100 BsAbs at various stage of clinical trials and three BsAbs with different modes of action have been approved.
Challenges
There are a number of challenges in the development and validation of immunogenicity assays (ADA assays) for bispecifics.
- Bispecifics need immunogenicity assays including characterization against the individual domains as per regulatory requirement
- It is important to have the right tools (positive control (PC) and monospecific or domain-specific “drugs” to characterize)
- Validation is more complicated and time/resource consuming than for a standard monoclonal antibody therapeutic (mAb)
- Data is interpreted for ADA against whole molecule and each of the domains
Required tools
Bi-specific confirmatory approach.
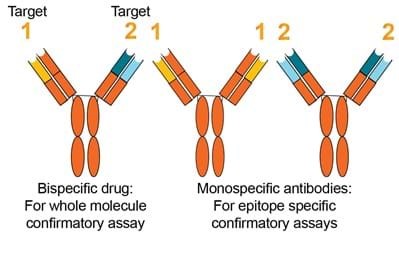
FDA 2019 Guidance (Section IV.A.3)
- An immune response to one domain may inhibit a specific function while leaving other intact. Direct initial screening and confirmatory tests against the whole therapeutic protein product recommended
- Examination of immune responses… such as bispecifics and ADCs may require development of multiple assays to measure immune responses to different domains of the molecules
EMA 2017 Guideline (Section 7.4)
- A strategy…to dissect the specificities of the antibodies to individual moieties may be used
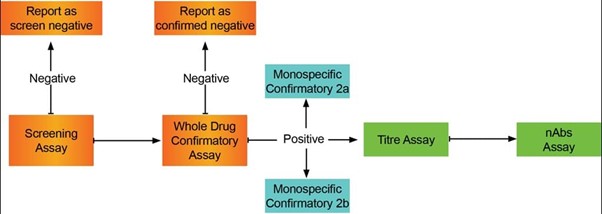
Bispecific ADA tiered approach
Immunogenicity assay approach for multi-domain proteins
Goal
The customer’s drug was a BsAb and they required an ADA assay to include domain-specific characterization.
Our solution
The customer was able to provide suitable positive controls against both arms of the bispecific along with versions of mono-specific drugs for each of the arms of the BsAbs.
We utilised the tools provided and our scientists, who have a wide range of expertise in development and validation of ADA assays, provided a solution.
Our process
Using a bridging assay format with acid pre-treatment to improve drug tolerance, DDS developed a screening assay and three different confirmatory assays. The ADA response was characterized against the whole drug, and against each of the monospecific drug arms.
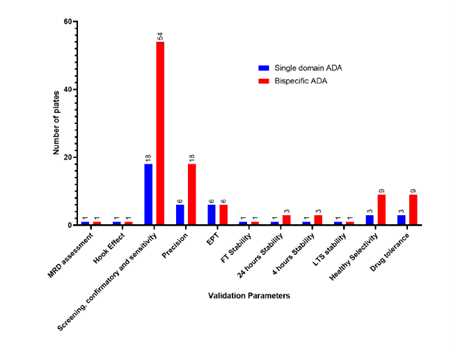
Single vs bispecific (Plates)
Total number of plates:
Single domain assay: ~42 plates
Bispecific domain assay: ~106 plates
Results
The validated assays will support sample analysis throughout the method lifecycle.
The customer is assured that regulatory requirements for a sensitive, selective, specific and drug tolerant assay have been met in order to support submissions.

